Below are the general guidelines for dosing clonidine. Note that these dosages may be adjusted on a case-by-case basis for individual patients. Always follow your prescribing physician’s instructions for taking clonidine.
The following information comes from DailyMed, an FDA label information provider.
What if I miss a dose of clonidine?
Mayo Clinic states to take the missed dose of clonidine as soon as possible, unless you are closer in time to your next available dose. You should never double doses. If you miss more than one dose of clonidine, Mayo Clinic states you should contact your prescribing physician immediately for instruction to avoid the serous risks of hypertension that could occur. If clonidine is not tapered off then patients are at risk of experiencing significant hypertension.
What if I overdose on clonidine?
**If you believe you or someone you know has overdosed, call 911 immediately for medical assistance.
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis. The frequency of CNS depression may be higher in children than adults. Large overdoses may result in reversible cardiac conduction defects or dysrhythmias, apnea, coma and seizures. Signs and symptoms of overdose generally occur within 30 minutes to two hours after exposure. As little as 0.1 mg of clonidine has produced signs of toxicity in children.
There is no specific antidote for clonidine overdosage. Clonidine overdosage may result in the rapid development of CNS depression; therefore, induction of vomiting with ipecac syrup is not recommended. Gastric lavage may be indicated following recent and/or large ingestions. Administration of activated charcoal and/or a cathartic may be beneficial. Supportive care may include atropine sulfate for bradycardia, intravenous fluids and/or vasopressor agents for hypotension and vasodilators for hypertension. Naloxone may be a useful adjunct for the management of clonidine-induced respiratory depression, hypotension and/or coma; blood pressure should be monitored since the administration of naloxone has occasionally resulted in paradoxical hypertension. Tolazoline administration has yielded inconsistent results and is not recommended as first-line therapy. Dialysis is not likely to significantly enhance the elimination of clonidine.
The largest overdose reported to date involved a 28-year old male who ingested 100 mg of clonidine hydrochloride powder. This patient developed hypertension followed by hypotension, bradycardia, apnea, hallucinations, semicoma, and premature ventricular contractions. The patient fully recovered after intensive treatment. Plasma clonidine levels were 60 ng/ml after 1 hour, 190 ng/ml after 1.5 hours, 370 ng/ml after 2 hours, and 120 ng/ml after 5.5 and 6.5 hours. In mice and rats, the oral LD50 of clonidine is 206 and 465 mg/kg, respectively.
Dosage and Administration
Adults: The dose of clonidine hydrochloride must be adjusted according to the patient’s individual blood pressure response. The following is a general guide to its administration.
Initial Dose: 0.1 mg tablet twice daily (morning and bedtime). Elderly patients may benefit from a lower initial dose.
Maintenance Dose: Further increments of 0.1 mg per day may be made at weekly intervals if necessary until the desired response is achieved. Taking the larger portion of the oral daily dose at bedtime may minimize transient adjustment effects of dry mouth and drowsiness. The therapeutic doses most commonly employed have ranged from 0.2 mg to 0.6 mg per day given in divided doses. Studies have indicated that 2.4 mg is the maximum effective daily dose, but doses as high as this have rarely been employed.
Renal Impairment: Dosage must be adjusted according to the degree of impairment, and patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
How is clonidine supplied?
0.1 mg â Each orange, round tablet imprinted with 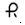 and 127 on one side and bisect on the other side contains 0.1 mg of Clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2127-10) and 500 (NDC 0228-2127-50).
and 127 on one side and bisect on the other side contains 0.1 mg of Clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2127-10) and 500 (NDC 0228-2127-50).
0.2 mg â Each orange, round tablet imprinted with 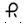 on one side and 128 and bisect on the other side contains 0.2 mg of Clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2128-10) and 500 (NDC 0228-2128-50).
on one side and 128 and bisect on the other side contains 0.2 mg of Clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2128-10) and 500 (NDC 0228-2128-50).
0.3 mg â Each orange, round tablet imprinted with 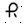 on one side and 129 and bisect on the other side contains 0.3 mg of Clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2129-10).
on one side and 129 and bisect on the other side contains 0.3 mg of Clonidine hydrochloride USP and is supplied in bottles of 100 (NDC 0228-2129-10).
Dispense in a tight, light-resistant container as defined in the USP.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Manufactured by:
Actavis Elizabeth LLC
200 Elmora Avenue
Elizabeth, NJ 07207 USA
Disclaimer: this article does not constitute or replace medical advice. If you have an emergency or a serious medical question, please contact a medical professional or call 911 immediately. To see our full medical disclaimer, visit our Terms of Use page.





