Below are the general guidelines for dosing Stelara (ustekinumab). Note that these dosages may be adjusted on a case-by-case basis for individual patients. Always follow your prescribing physician’s instructions for taking Stelara.
The following information comes from DailyMed, an FDA label information provider.
What if I miss a dose of Stelara?
This medicine needs to be given on a fixed schedule. If you miss a dose or forget to use your medicine, call your doctor or pharmacist for instructions.
What if I overdose on Stelara?
Single doses up to 6 mg/kg intravenously have been administered in clinical studies without dose-limiting toxicity. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment be instituted immediately.
How is Stelara administered?
Psoriasis
Subcutaneous Adult Dosage Regimen
- For patients weighing 100 kg or less, the recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients weighing more than 100 kg, the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
In subjects weighing more than 100 kg, 45 mg was also shown to be efficacious. However, 90 mg resulted in greater efficacy in these subjects.
Subcutaneous Adolescent Dosage Regimen
Administer STELARA® subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of STELARA® for adolescents (12-17 years old) based on body weight is shown below (Table 1).
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
| less than 60 kg | 0.75 mg/kg |
| 60 kg to 100 kg | 45 mg |
| more than 100 kg | 90 mg |
For adolescent patients weighing less than 60 kg, the administration volume for the recommended dose (0.75 mg/kg) is shown in Table 2; withdraw the appropriate volume from the single-dose vial.
| Body Weight (kg) at the time of dosing | Dose (mg) | Volume of injection (mL) |
|---|---|---|
| 30 | 22.5 | 0.25 |
| 31 | 23.3 | 0.26 |
| 32 | 24 | 0.27 |
| 33 | 24.8 | 0.27 |
| 34 | 25.5 | 0.28 |
| 35 | 26.3 | 0.29 |
| 36 | 27 | 0.3 |
| 37 | 27.8 | 0.31 |
| 38 | 28.5 | 0.32 |
| 39 | 29.3 | 0.32 |
| 40 | 30 | 0.33 |
| 41 | 30.8 | 0.34 |
| 42 | 31.5 | 0.35 |
| 43 | 32.3 | 0.36 |
| 44 | 33 | 0.37 |
| 45 | 33.8 | 0.37 |
| 46 | 34.5 | 0.38 |
| 47 | 35.3 | 0.39 |
| 48 | 36 | 0.4 |
| 49 | 36.8 | 0.41 |
| 50 | 37.5 | 0.42 |
| 51 | 38.3 | 0.42 |
| 52 | 39 | 0.43 |
| 53 | 39.8 | 0.44 |
| 54 | 40.5 | 0.45 |
| 55 | 41.3 | 0.46 |
| 56 | 42 | 0.46 |
| 57 | 42.8 | 0.47 |
| 58 | 43.5 | 0.48 |
| 59 | 44.3 | 0.49 |
Psoriatic Arthritis
Subcutaneous Adult Dosage Regimen
- The recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients with co-existent moderate-to-severe plaque psoriasis weighing more than 100 kg, the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
Crohn’s Disease and Ulcerative Colitis
Intravenous Induction Adult Dosage Regimen
A single intravenous infusion dose of STELARA® dosage regimen specified in Table 3.
| Body Weight of Patient at the time of dosing | Dose | Number of 130 mg/26 mL (5 mg/mL) STELARA® vials |
|---|---|---|
| 55 kg or less | 260 mg | 2 |
| more than 55 kg to 85 kg | 390 mg | 3 |
| more than 85 kg | 520 mg | 4 |
Subcutaneous Maintenance Adult Dosage Regimen
The recommended maintenance dosage is a subcutaneous 90 mg dose administered 8 weeks after the initial intravenous dose, then every 8 weeks thereafter.
General Considerations for Administration
- STELARA® is intended for use under the guidance and supervision of a physician. STELARA® should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician. The appropriate dose should be determined by a healthcare provider using the patient’s current weight at the time of dosing. In adolescent patients, orphan drug designation has been granted and it is recommended that STELARA® be administered by a healthcare provider. If a physician determines that it is appropriate, a patient may self-inject or a caregiver may inject STELARA® after proper training in subcutaneous injection technique. Patients should be instructed to follow the directions provided in the Medication Guide.
- The needle cover on the prefilled syringe contains dry natural rubber (a derivative of latex). The needle cover should not be handled by persons sensitive to latex.
- It is recommended that each injection be administered at a different anatomic location (such as upper arms, gluteal regions, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, or indurated. When using the single-dose vial, a 1 mL syringe with a 27 gauge, ½ inch needle is recommended.
- Prior to administration, visually inspect STELARA® for particulate matter and discoloration. STELARA® is a colorless to the light yellow solution and may contain a few small translucent or white particles. Do not use STELARA® if it is discolored or cloudy, or if another particulate matter is present. STELARA® does not contain preservatives; therefore, discard any unused product remaining in the vial and/or syringe.
Instructions for Administration of STELARA® Prefilled Syringes Equipped with Needle Safety Guard
Refer to the diagram below for the provided instructions.
To prevent premature activation of the needle safety guard, do not touch the NEEDLE GUARD ACTIVATION CLIPS at any time during use.
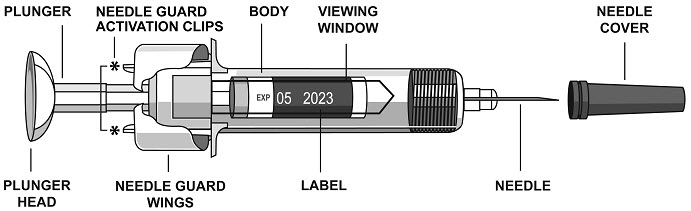
- Hold the BODY and remove the NEEDLE COVER. Do not hold the PLUNGER or PLUNGER HEAD while removing the NEEDLE COVER or the PLUNGER may move. Do not use the prefilled syringe if it is dropped without the NEEDLE COVER in place.
- Inject STELARA® subcutaneously as recommended.
- Inject all of the medication by pushing in the PLUNGER until the PLUNGER HEAD is completely between the needle guard wings. Injection of the entire prefilled syringe contents is necessary to activate the needle guard.

- After injection, maintain the pressure on the PLUNGER HEAD and remove the needle from the skin. Slowly take your thumb off the PLUNGER HEAD to allow the empty syringe to move up until the entire needle is covered by the needle guard, as shown by the illustration below:

- Used syringes should be placed in a puncture-resistant container.
Preparation and Administration of STELARA® 130 mg/26 mL (5 mg/mL) Vial for Intravenous Infusion (Crohn’s Disease and Ulcerative Colitis)
STELARA® solution for intravenous infusion must be diluted, prepared and infused by a healthcare professional using aseptic technique.
- Calculate the dose and the number of STELARA® vials needed based on patient weight (Table 3). Each 26 mL vial of STELARA® contains 130 mg of ustekinumab.
- Withdraw, and then discard a volume of the 0.9% Sodium Chloride Injection, USP from the 250 mL infusion bag equal to the volume of STELARA® to be added (discard 26 mL sodium chloride for each vial of STELARA® needed, for 2 vials- discard 52 mL, for 3 vials- discard 78 mL, 4 vials- discard 104 mL). Alternatively, a 250 mL infusion bag containing 0.45% Sodium Chloride Injection, USP may be used.
- Withdraw 26 mL of STELARA® from each vial needed and add it to the 250 mL infusion bag. The final volume in the infusion bag should be 250 mL. Gently mix.
- Visually inspect the diluted solution before infusion. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
- Infuse the diluted solution over a period of at least one hour. Once diluted, the infusion should be completely administered within eight hours of the dilution in the infusion bag.
- Use only an infusion set with an in-line, sterile, non-pyrogenic, low protein-binding filter (pore size 0.2 micrometer).
- Do not infuse STELARA® concomitantly in the same intravenous line with other agents.
- STELARA® does not contain preservatives. Each vial is for single use only. Discard any remaining solution. Dispose any unused medicinal product in accordance with local requirements.
Storage
If necessary, the diluted infusion solution may be kept at room temperature up to 25°C (77°F) for up to 4 hours. Storage time at room temperature begins once the diluted solution has been prepared. The infusion should be completed within 8 hours after the dilution in the infusion bag (cumulative time after preparation including the storage and the infusion period). Do not freeze. Discard any unused portion of the infusion solution.
Disclaimer: this article does not constitute or replace medical advice. If you have an emergency or a serious medical question, please contact a medical professional or call 911 immediately. To see our full medical disclaimer, visit our Terms of Use page.